|
Kinetics of gas adsorption on carbon nanotubes |
|
1. Why study experimentally the kinetics of adsorption on SWNTs? |
|
Fundamental: |
|
- Because it is a “new” problem, one for which there now are theoretical results that can be tested. - Because by studying adsorption kinetics we might understand why it is that the adsorption results that have been reported on open-ended tubes are so widely different (in contrast to those for close-ended tubes, for which a consensus view has now developed). |
|
Practical: |
|
- Because before adsorption on carbon nanotubes can receive wide use in any application, the time scales involved in adsorption processes need to be studied so as to provide limits or ranges within which such applications may develop. |
|
2. What kind of adsorption kinetics? |
|
-Not adsorption kinetic measurements in the sense of surface physics (i.e., involving ultra high vacuum and the determination of sticking coefficients) -Instead, more of a “chemical-engineering-like” version (i.e., involving the behavior of gas on a sorbing bed as a function of time, pressure and loading). |
|
3. Why use this approach? |
|
-Because the fundamental questions regarding adsorption on open-ended tubes can be approached within this context. -Because any practical application of adsorption on nanotubes will occur within this context, as well. |
|
4. How adsorption kinetics are measured? |
|
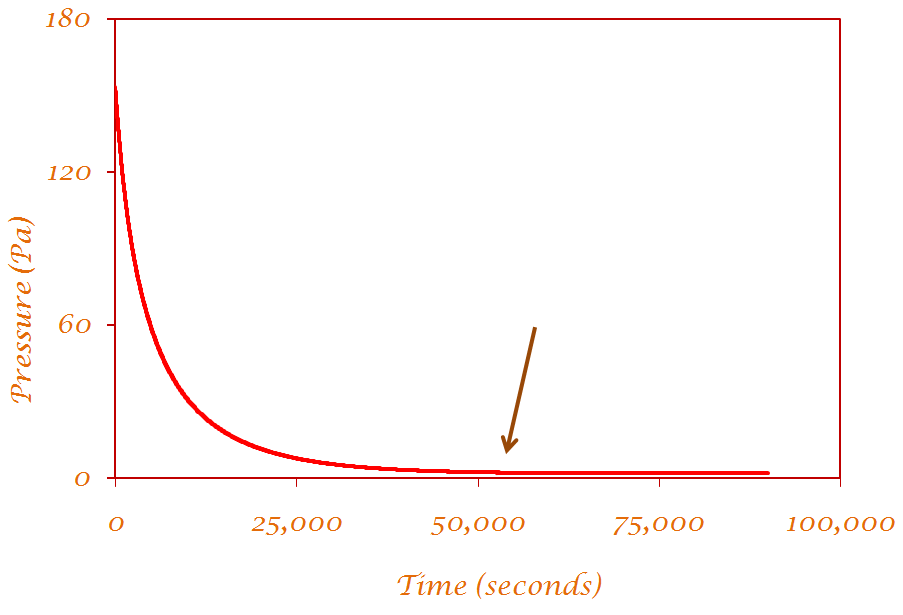
The kinetic measurements are conducted for each point along an adsorption isotherm. To measure a data point in an adsorption isotherm, a known amount of gas is added to a cell at a controlled temperature. Then, the pressure of the gas in the cell is measured until its value reaches equilibrium. The amount adsorbed at this equilibrium pressure is then determined. In these kinetic measurements what we monitor is the evolution of the pressure in the cell (or, equivalently, of the amount adsorbed on the substrate) as a function of time after the gas is added to the cell. Our determination of equilibrium, for each point, consists of establishing when, in a plot of the pressure in the cell as a function of time evolved since dosing, does the plot become parallel to the x-axis (time evolved). |
|
|
|
The time it takes for equilibrium to be reached is the equilibration time. |
|
Experimental results |
|
Dinesh S. Rawat, M. Mercedes Calbi, Aldo D. Migone, “Equilibration Time: Kinetics of Gas adsorption on closed and open-ended Single Walled Carbon Nanotubes”, J. Phys. Chem. C., 12980-12986 (2007). |
|
D. S Rawat; V. Krungleviciute; L. Heroux; M. Bulut; M. M. Calbi and A. D. Migone, “Dependence of single-walled carbon nanotubes’ adsorption kinetics on temperature and binding energy“; accepted for publication in Langmuir (available online). |
|
Computer simulation results obtained by Prof. M. Mercedes Calbi are published in the following article: |
|
"Physisorption Kinetics in Carbon Nanotube Bundles" Burde, J. T.; Calbi, M. M. J. Phys. Chem. C.; 2007; 111(13); 5057-5063. |
|
• |
|
Last Updated November 30, 2008 |