|
Metal-organic frameworks
|
|
Metal-organic frameworks |
|
1D, 2D, or 3D porous structures Network of metal ions linked by organic ligands Well defined, perfectly ordered channels and uniform pores with a small pore dimension (<2nm) and high micropore volume and surface area Organic ligands allow the structures to be flexible Ligands can be changed to modify the pore structure allowing access to specific gases Metals may be selected to increase sorbent-sorbate interactions Synthesis is highly reproducible and cost effective while still being high purity |
|
Samples |
|
1. Cu-BTC metal-organic framework |
|
a) Cu-BTC metal-organic framework - 3D cubic network (Fm-3m) |
 |
|
Cu is shown in green, O is shown in red, C is shown in gray (H is omitted for clarity). |
|
Figure provided by Prof. Jing Li (Rutgers University) |
|
b) Preparation of Cu-BTC metal-organic framework |
|
1. Cu(NO3)2·3H2O (98% purity) dissolved in de-ionized water 2. Trimesic acid (BTC) (95% purity) dissolved in ethanol 3. Reaction of Cu(NO3)2·3H2O and BTC in a Parr reactor at 403 K for 12 hours 4. Turquoise crystals formed in high yield 5. Heated for further purification at 100° C for 6 hours |
|
2. RPM1-Co metal-organic framework |
|
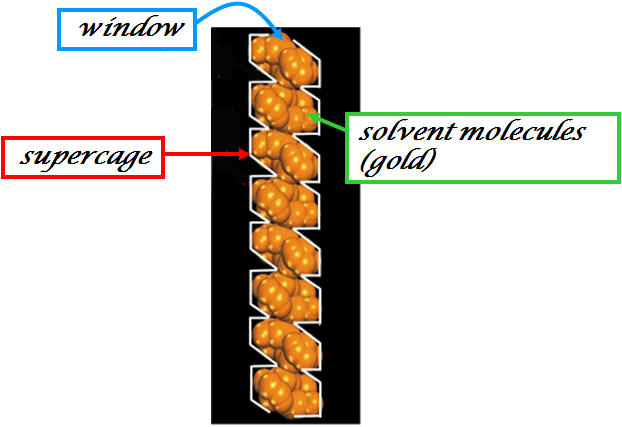
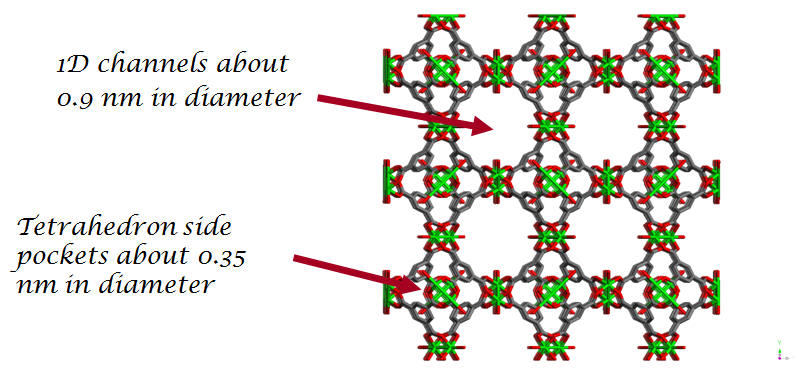
RPM1-Co - Rutgers Recyclable Porous Materials RPM1-Co - [Co3(bpdc)3(bpy)]·4DMF·H2O |
|
|
|
Side view (100) of one-dimensional channel in RPM-1-Co |
|
Figure provided by Prof. Jing Li (Rutgers University) |
|
Experimental results |
|
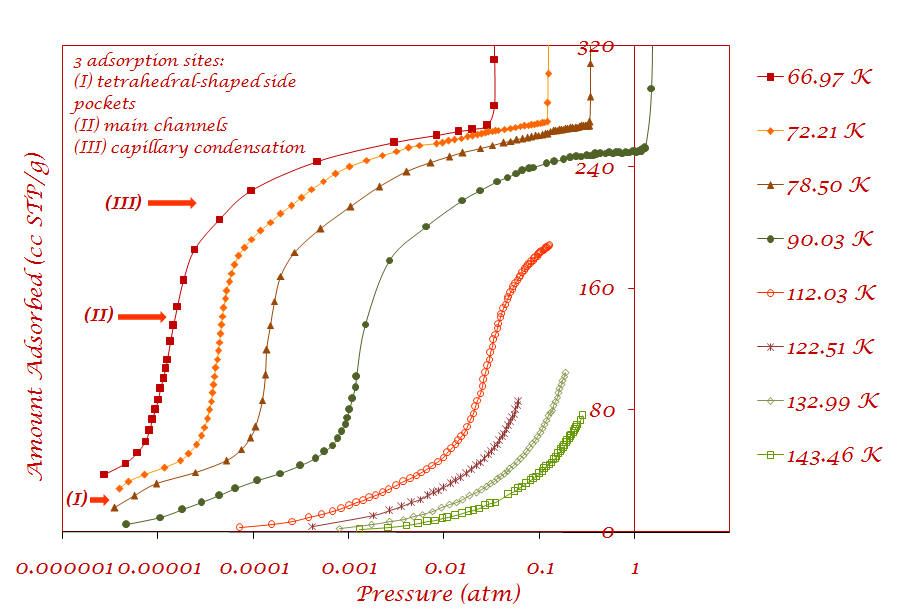
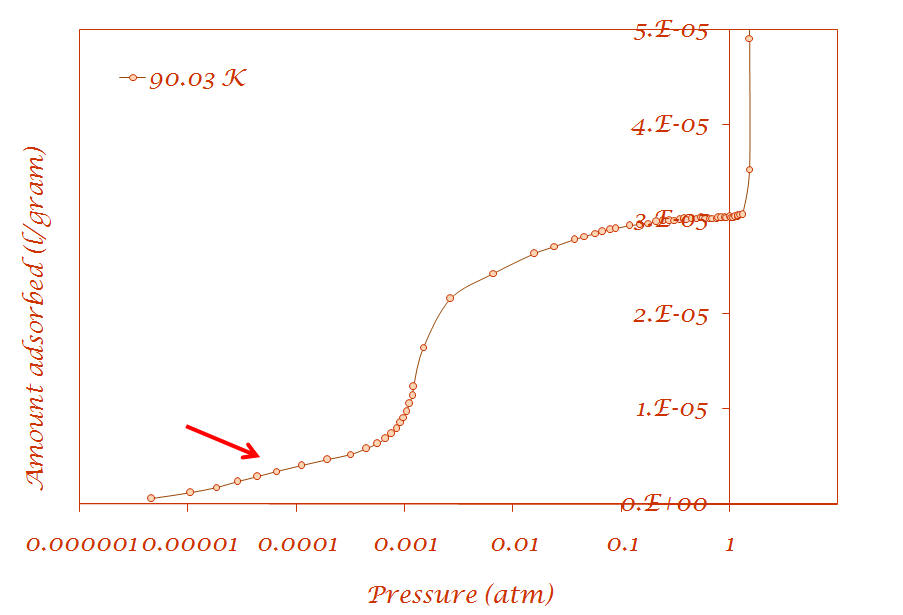
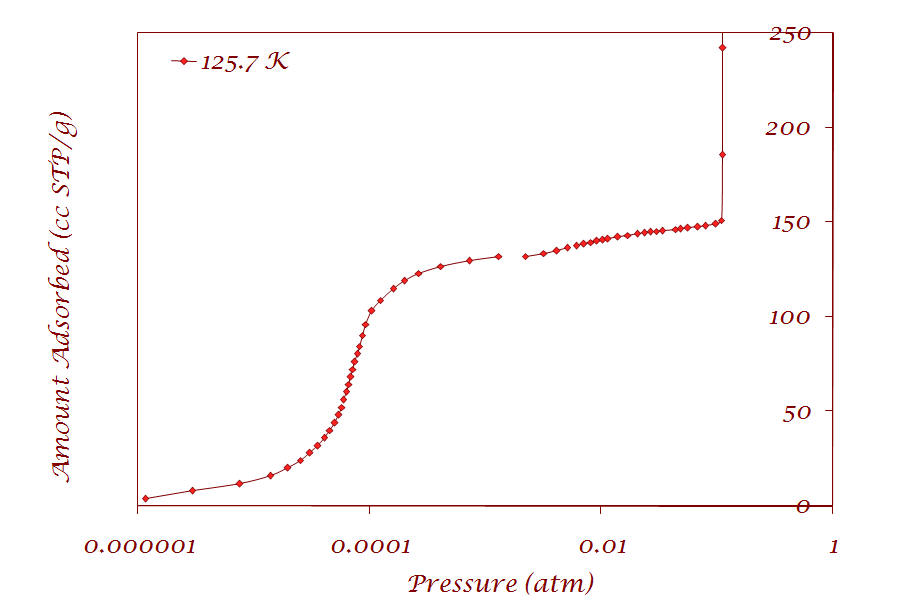
1. Argon adsorption on Cu-BTC metal-organic framework (Sample provided by Prof. Jing Li - Rutgers University) |
|
Results are published in the following article: V. Krungleviciute, K. Lask, L. Heroux, A. D. Migone, J.-Y. Lee, J. Li, A. Skoulidas, “Argon adsorption on Cu3(BTC)2(H2O)3 (BTC=Benzene-1,3,5-tricarboxylate) metal-organic framework”, Langmuir, 23; 3106-3109 (2007) |
|
2. Gas separation using RPM1-Co and Cu-BTC metal-organic frameworks (Sample provided by Prof. Jing Li - Rutgers University) |
|
Results are published in the following article: |
|
V. Krungleviciute, K. Lask, A. D. Migone, J.-Y. Lee, J. Li, "Kinetics and equilibrium of gas adsorption on RPM1-Co and Cu-BTC metal-organic frameworks: potential for gas separation applications", AIChE J. 54 918-923 (2008) |
|
Categorization of adsorptive separation processes based on mechanism of separation |
|
There are 3 different mechanisms through which adsorptive gas separation can be achieved. In our article we discuss 2 of them. |
|
a) Steric – only certain size and shape molecules diffuse into the pores of the adsorbent. |
|
The substep present in the Ar adsorption isotherm that corresponds to adsorption in the tetrahedral-shaped side pockets is absent in the CF4 isotherm. This shows that only the smaller and properly shaped argon molecule fits in the pores while the CF4 molecule is too big to diffuse into the side-pockets. This is an example of the steric separation mechanism. |
|
Argon adsorption on Cu-BTC MOFs |
|
|
|
CF4 adsorption on Cu-BTC MOFs |
|
|
|
b) Kinetic – some of the gases adsorb on the surface or diffuse into pores of the substrate faster than other ones. |
|
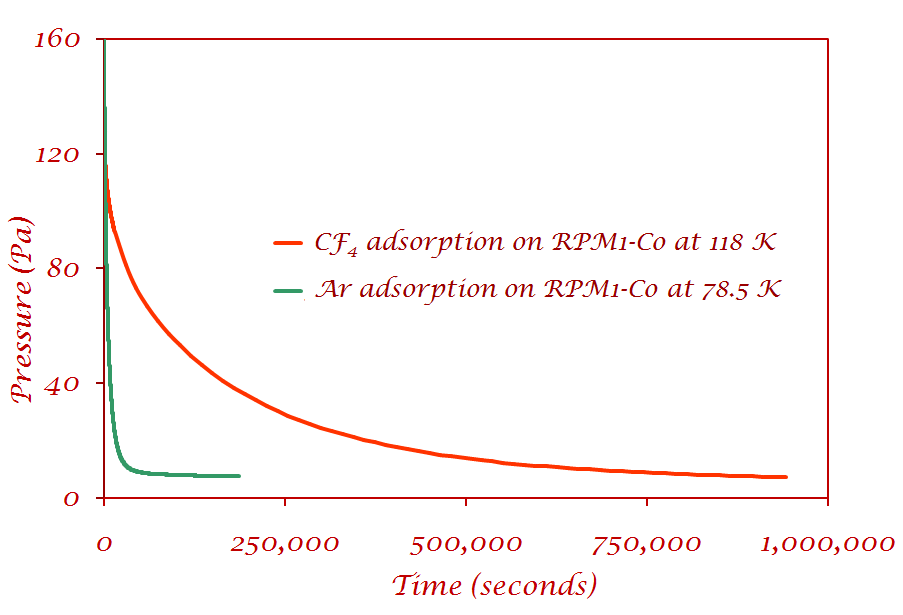
I) Pressure decrease as a function of time for Ar and CF4 on RPM1-Co |
|
CF4 - the pressure had not reached equilibrium even after eleven days Ar - reaches equilibrium at least an order of magnitude faster than CF4 No leaks were present, as was verified by measuring how much gas desorbed from the sample.
|
|
|
|
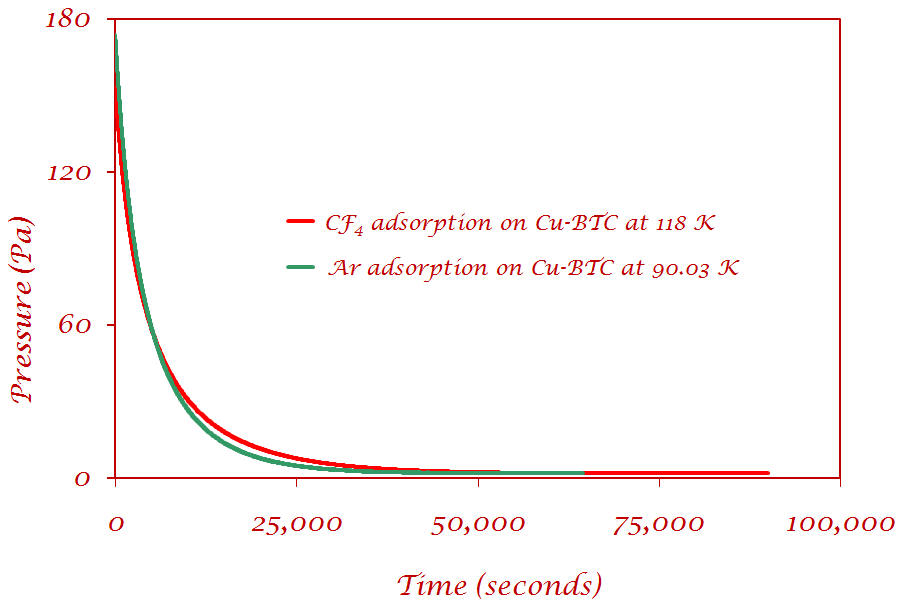
II) Pressure decrease as a function of time for Ar and CF4 on Cu-BTC |
|
CF4 and Ar - the pressure reaches equilibrium around the same time (~50,000 seconds) |
|
|
|
• |
|
Last Updated November 30, 2008 |