Main Content Area
Facilities and Equipments
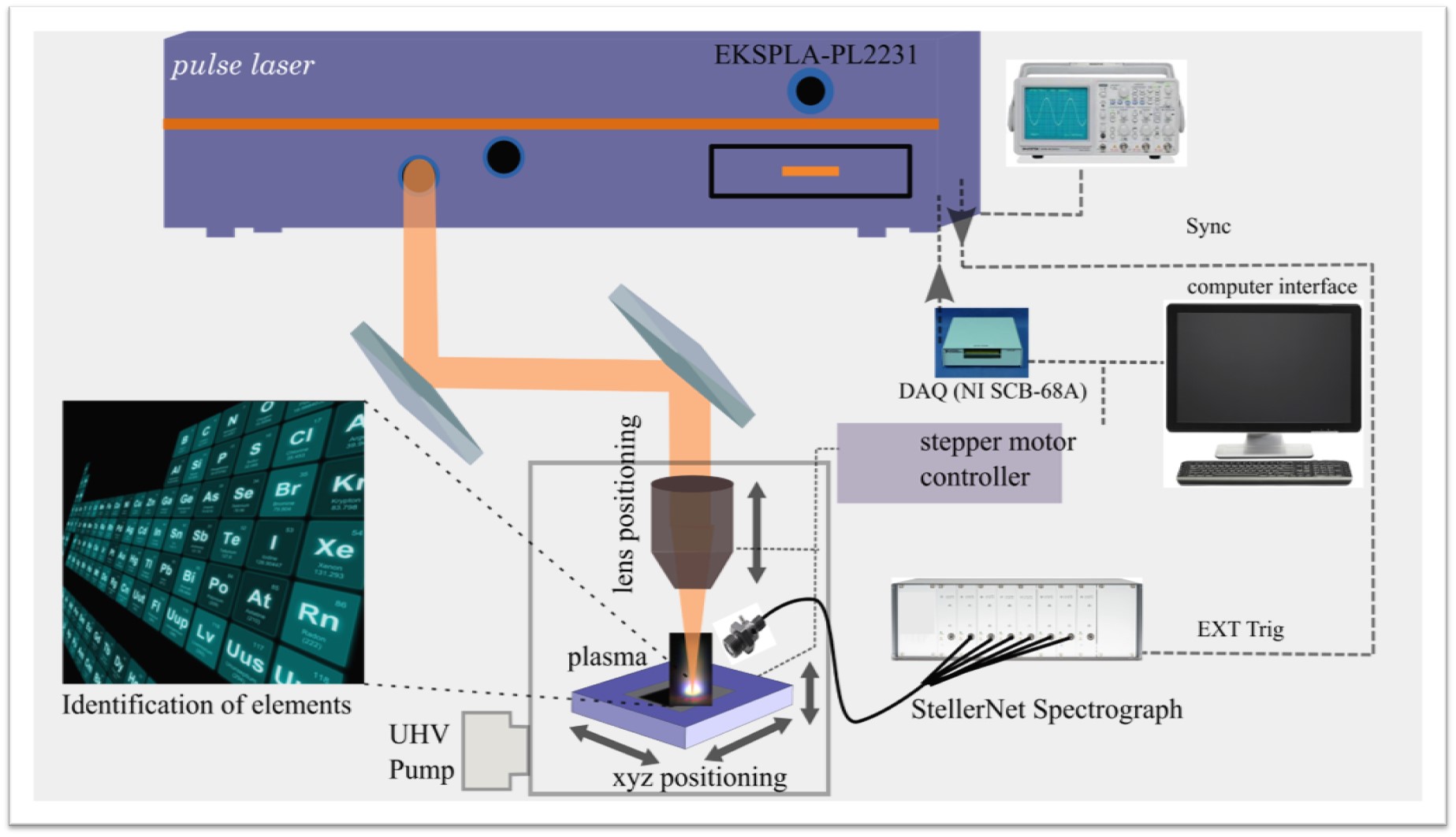
Laser Induced Breakdowns Spectroscopy (LIBS)
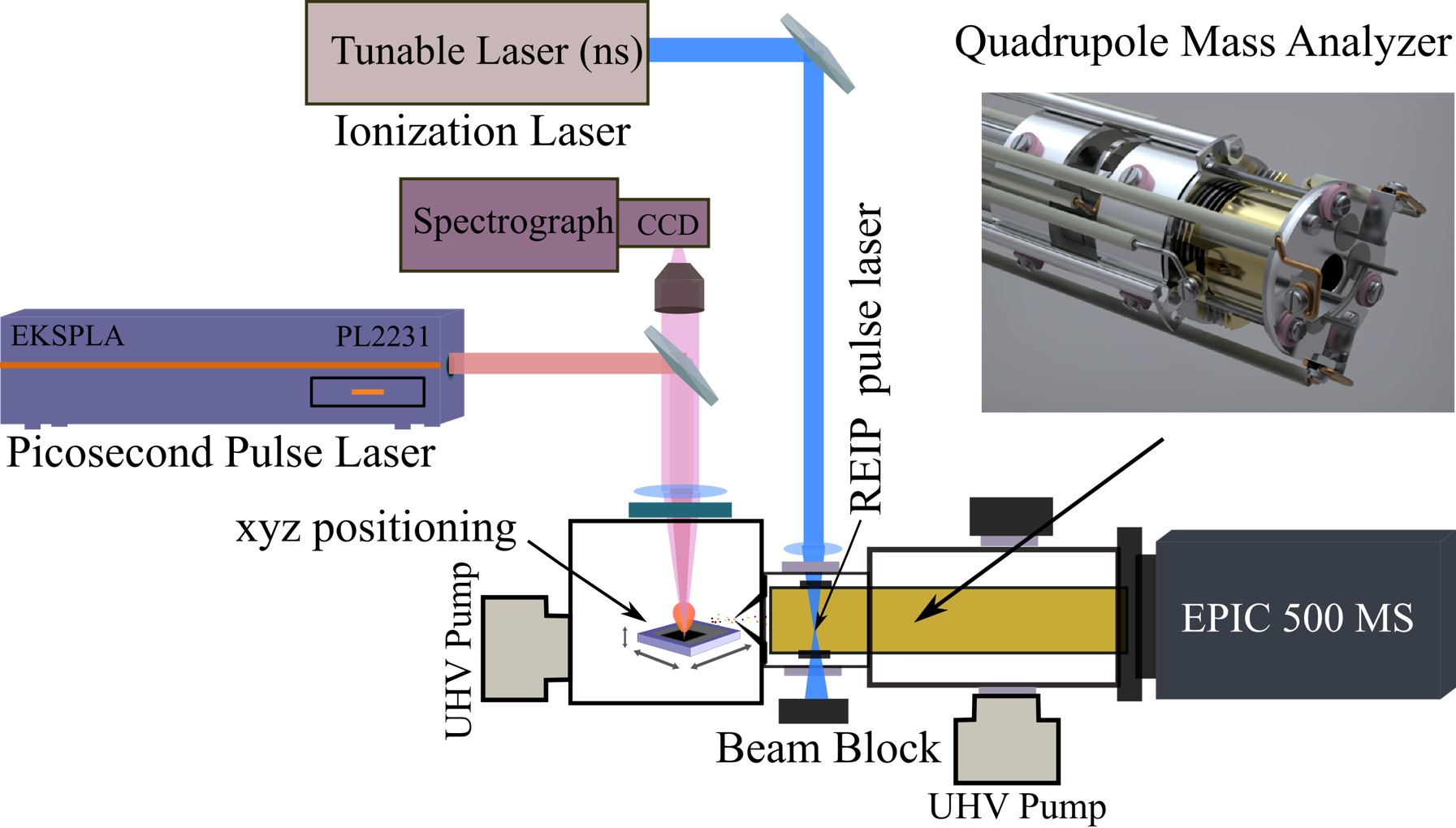
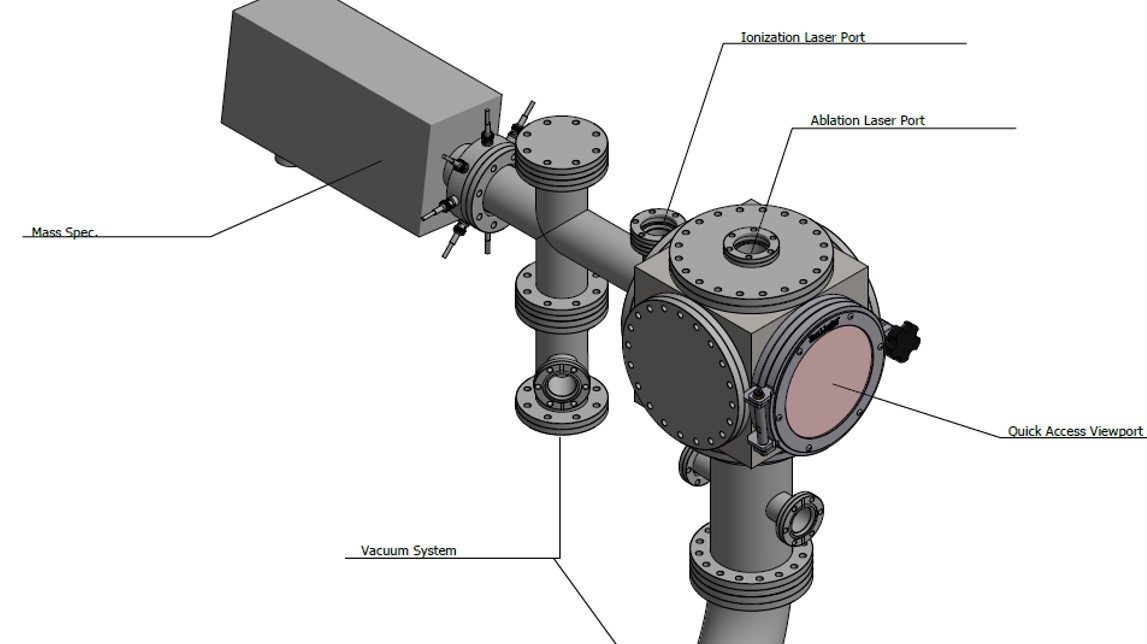
Laser Ablation-Resonance Enhanced Photoionization Mass Spectrometry (LA-REPMS)
Mass spectrometry is one of the most powerful analytical techniques across various disciplines, especially for probing elements and molecules in complex media. It utilizes the mass-to-charge ratio of charged species in an electric or magnetic field to detect elemental or molecular constituents in complex media. The targeted chemical components need to be ionized, which are then sorted and separated according to the mass-to-charge ratio. Therefore, a method of ionization is a crucial step in improving the performance of mass spectrometry. Innovative ionization methods and spatial and depth characterization allow us to obtain significantly improved sensitivity and selectivity. In this regard, we seek to adopt Laser Ablation (LA) with Resonance Enhanced Ionization (REI) in mass spectrometry to increase the detection capability of elements and molecules in a complex media with spatial and depth characterization. Unlike other methods, this approach mitigates the interference of the chemical and physical nature of the sample by a selective ionization process using a tunable laser and increasing the sensitivity and selectivity. Furthermore, the EPIC-500 mass spectrometer has the flexibility of using photons or electrons in impact ionization that enables us to monitor entire atomic and molecular species simultaneously or analyze a key element, molecule, or isotope with ultimate sensitivity. The ionization technique can deliver a better signal to noise ratio for optimal detection limits and reproducibility for nearly every element in the periodic table and even small molecules in complex matrices over typical mass spectrometry, such as ICP-MS. This innovative ionization method enhances our ability to see and monitor critical peaks (such as isotopes) and allows us to access additional information for better characterization.
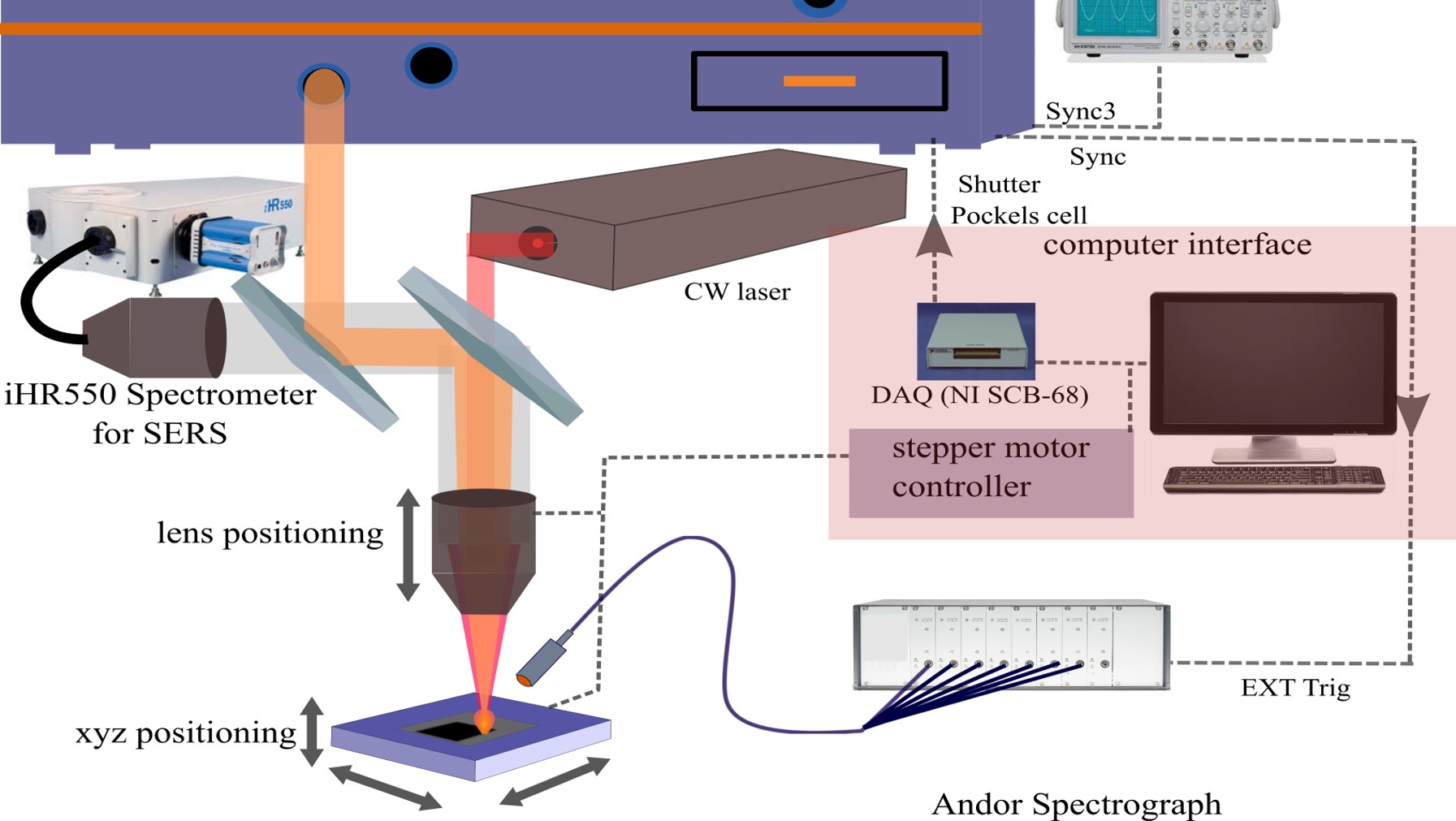
Surface-enhanced Raman Spectroscopy (SERS)
Chemical structure and composition of the sample are identified, quantified, and distinguished from others via measuring the vibrational energy levels of chemical bonds using Raman Scattering in which a powerful analytical tool for chemical analysis. These methods are more convenient for any application because they require little to no sample preparation, highly selective, nondestructive and in-situ analysis procedure. Photons from inelastic scattering are very sensitive to changes in molecular structure and are a fingerprint of the molecule. SERS is highly sensitive to slight changes at the molecular level, and it requires no sample preparation, which makes it suitable for a broad range of analytical applications. Compared to other techniques, SERS has advantages such as non-destruction of the sample during laser excitation. In this approach, near infrared (NIR, CW laser @ 785-nm) wavelength light is used as an excitation laser source to minimize the unwanted auto-fluorescence from biological samples, such as blood or serum. Wavelength shift from inelastic scattering radiation, which provides the chemical and structural information of samples, is collected by an objective lens and coupled to a high-resolution spectrometer via a fiber-optic interface. To measure the small fraction of scattered light from the sample, a thermo-electrically cooled high sensitive and low noise high-speed charged-coupled device (CCD) will be used. In the future, the spectrometer will be upgraded with a high sensitivity InGaAs array detector for an IR-Raman in which reduce the auto-fluorescence from biomedical samples greatly.
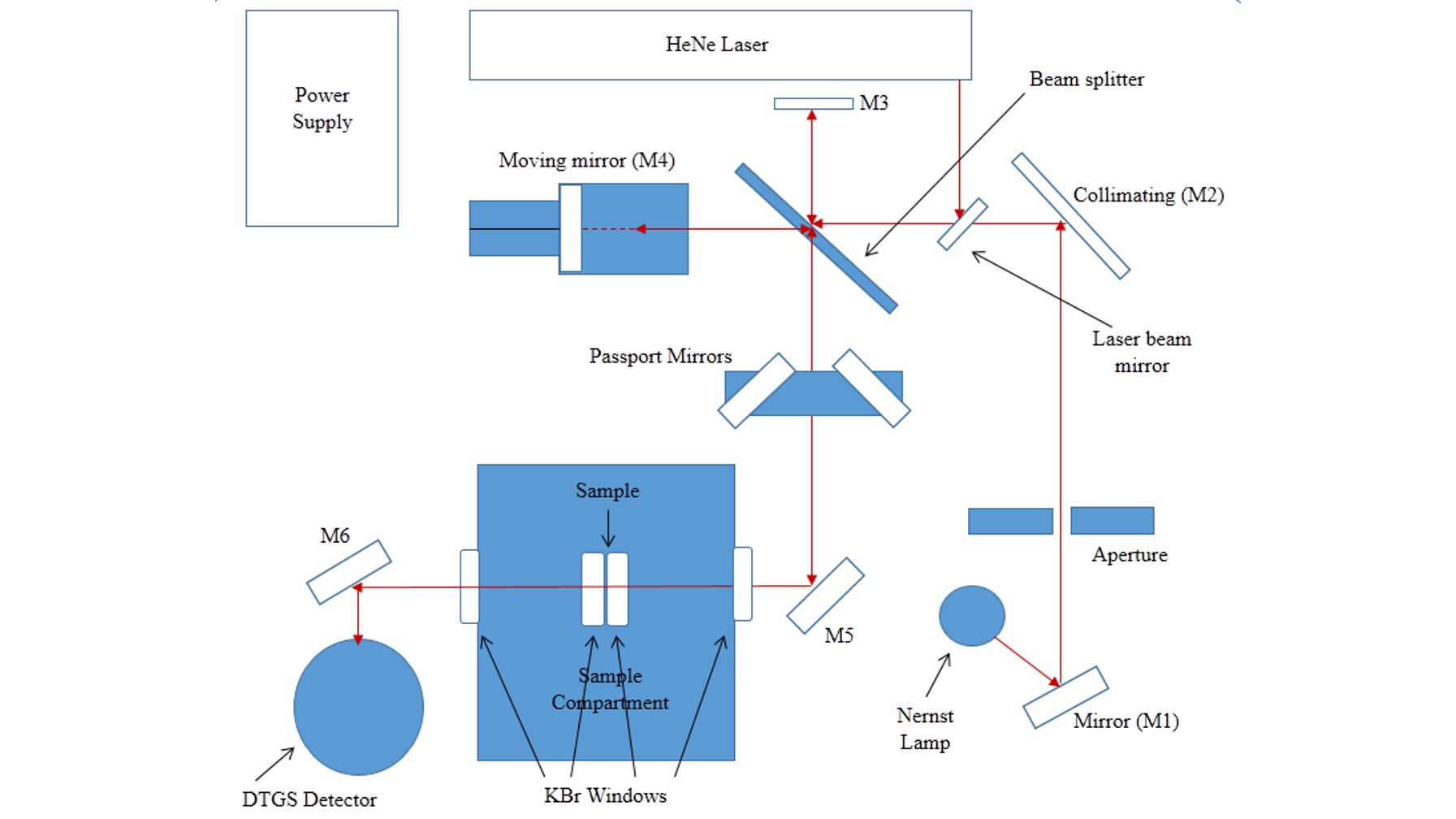
Fourier Transformed-infrared Spectroscopy (FT-IR)
FT-IR is an infrared (IR) spectroscopy, one of the most common and widely used spectroscopy techniques for chemical analysis, based on the Michelson interferometer technique. Unlike a disperse spectroscopy technique, this collects all the wavelength simultaneously using a Fourier transformation method. Chemical information of the sample can be identified via vibrational energy levels of molecules. Absorption of IR radiation triggers the vibrational excitation of an electron that leads to vibration (stretching, compressing, and bending) of molecular bonds. The net change in dipole moment in a molecule as it vibrates or rotates due to IR absorption becomes active for the FT-IR analysis. So it is possible to identify the molecule by comparing its vibrational frequency of an IR spectrum. FT-IR is a sensitive technique particularly for identifying most of the organic chemicals and some inorganic. In our facilities, we have a high resolution (0.1 cm -1 ) bench-top Thermo-Nicolet Nexus 670 FT-IR spectrometer, which can collect the spectra from 400 to 4000 cm-1 .
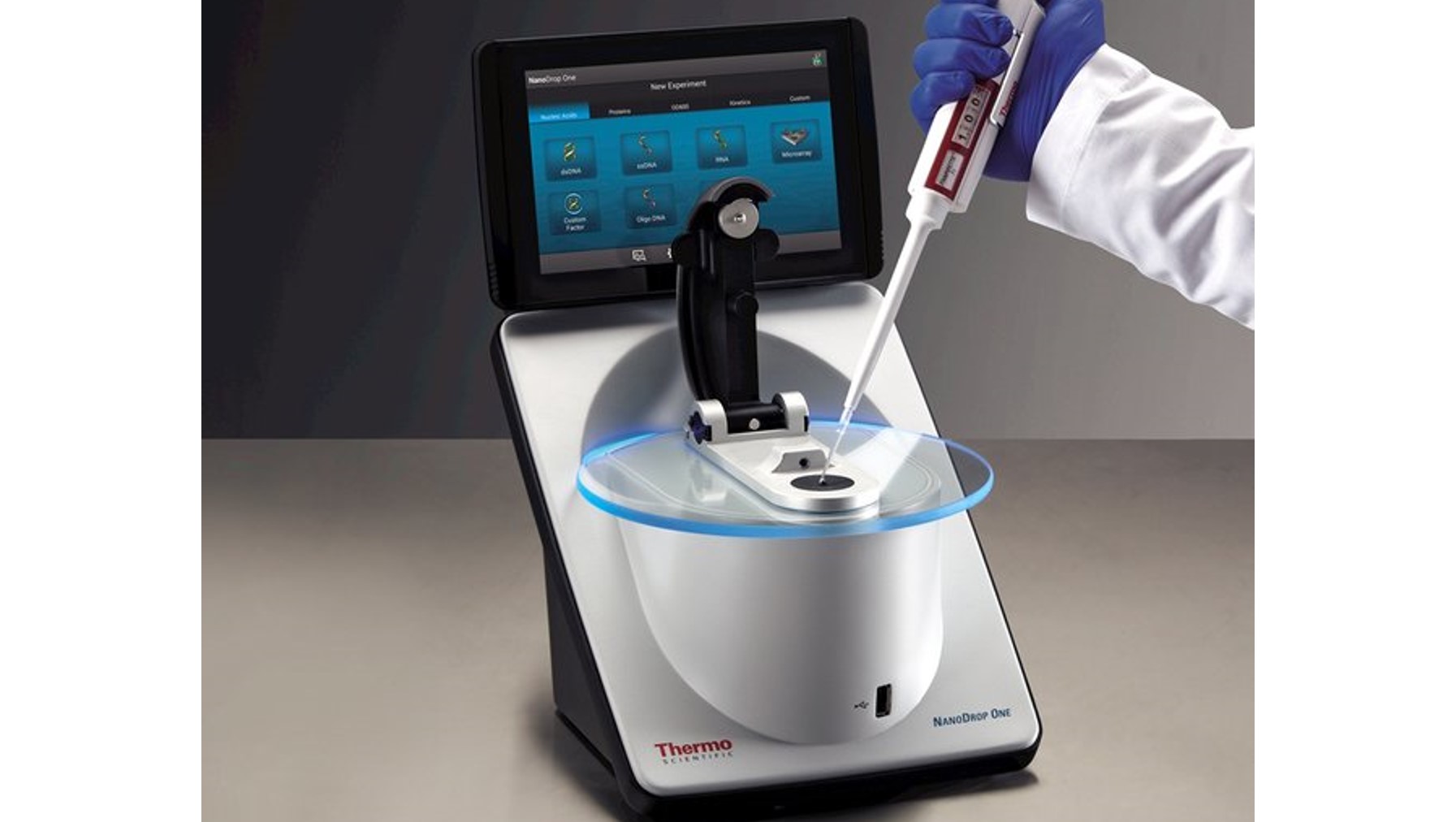
NanoDropTM One/Onec Microvolume UV-Vis Spectrophotometer
UV-Vis spectrophotometer from Thermo Scientific for a preliminary study of bio-samples such as nucleic acids and a wide variety of proteins before major experiment with a minimal sample (micro-volume) that enables analysis without the need for dilutions. This model also has the features for analysis dilute samples using standard UV-Vis cuvettes.
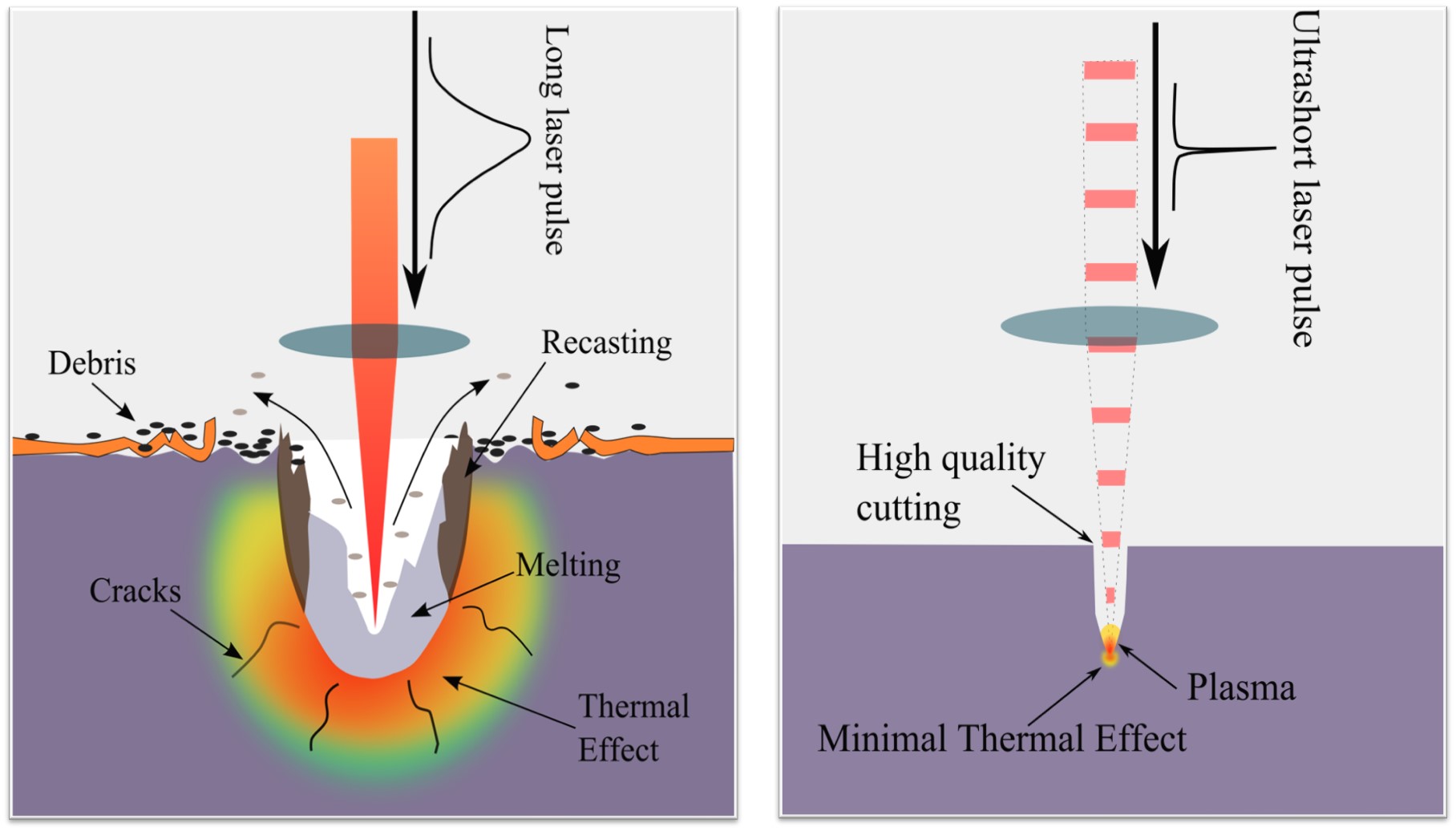
LIBS is an atomic emission spectroscopy technique based on laser-induced plasma generation. It is capable of determining the elemental concentration down to part-per-million (ppm) level, and t allows the local analysis in micro-regions in the order of tens microns spatial resolution typically. Further, it requires no or minimal sample preparation and much smaller volumes of samples. The fundamental aspect of LIBS is relatively straightforward, and it allows real-time spectrum analysis. In this method, typically, a micro-plasma is generated by ablating the sample with a short pulse laser operating at fundamental wavelength (1064 nm) focused on the sample surface. The formed plasma contains free electrons, atoms, ions, and molecules in different excited states When high-temperature energetic plasma starts to cool, the species at excited electronic states fall into the ground state and emit discrete spectral lines. The optical emissions from plasma are collected and identified using a high-resolution spectrograph. Ultra-short laser pulse, from a femtosecond to a picosecond regime, has several advantages over nanosecond laser pulse such as the high precision of sample removal, lower continuum background, and fast plasma dissipation. In addition, the fast energy deposition allows minimal thermal damages of the sample. In our facilities, ultra-short laser pulse duration in picosecond regimes (<30 ps) is used to ablate the sample. The optical emission will be analyzed by a high-resolution Steller Net, Inc 7-channel spectrograph which can detect from 200nm to 1200 nm spectral range.